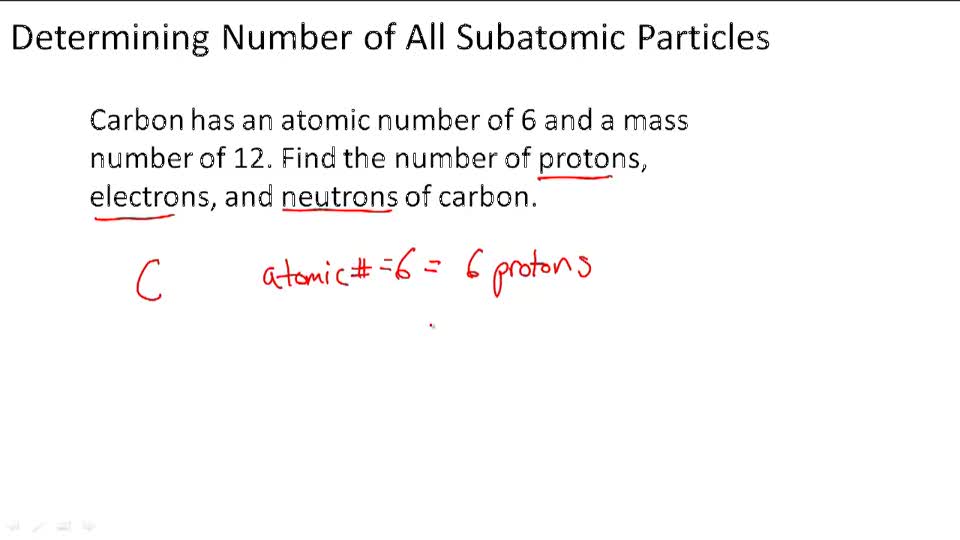
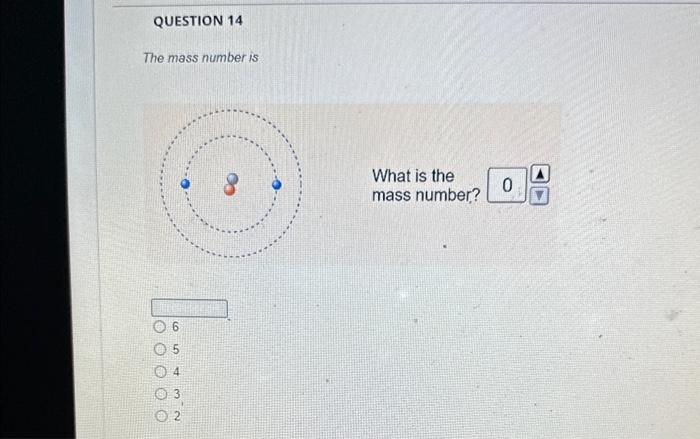
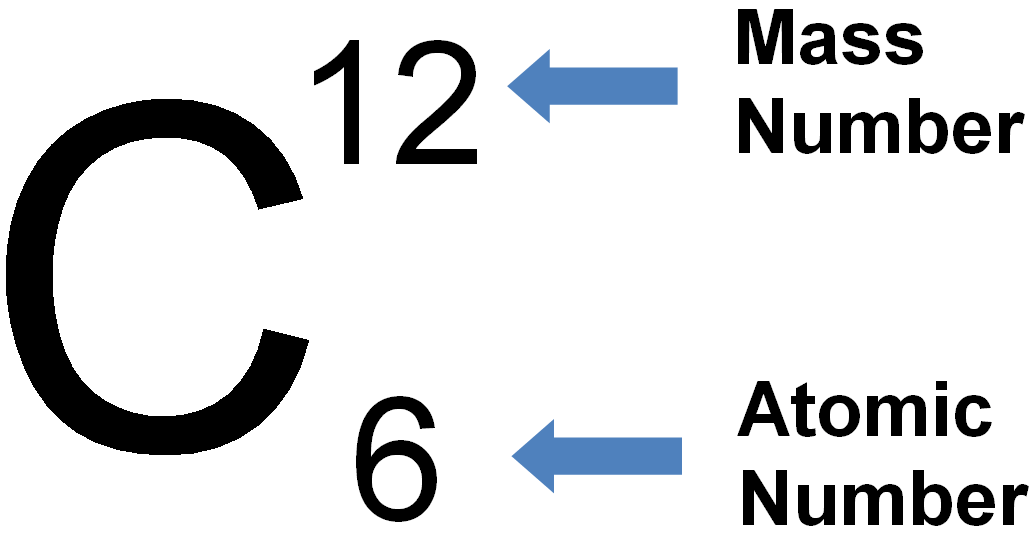
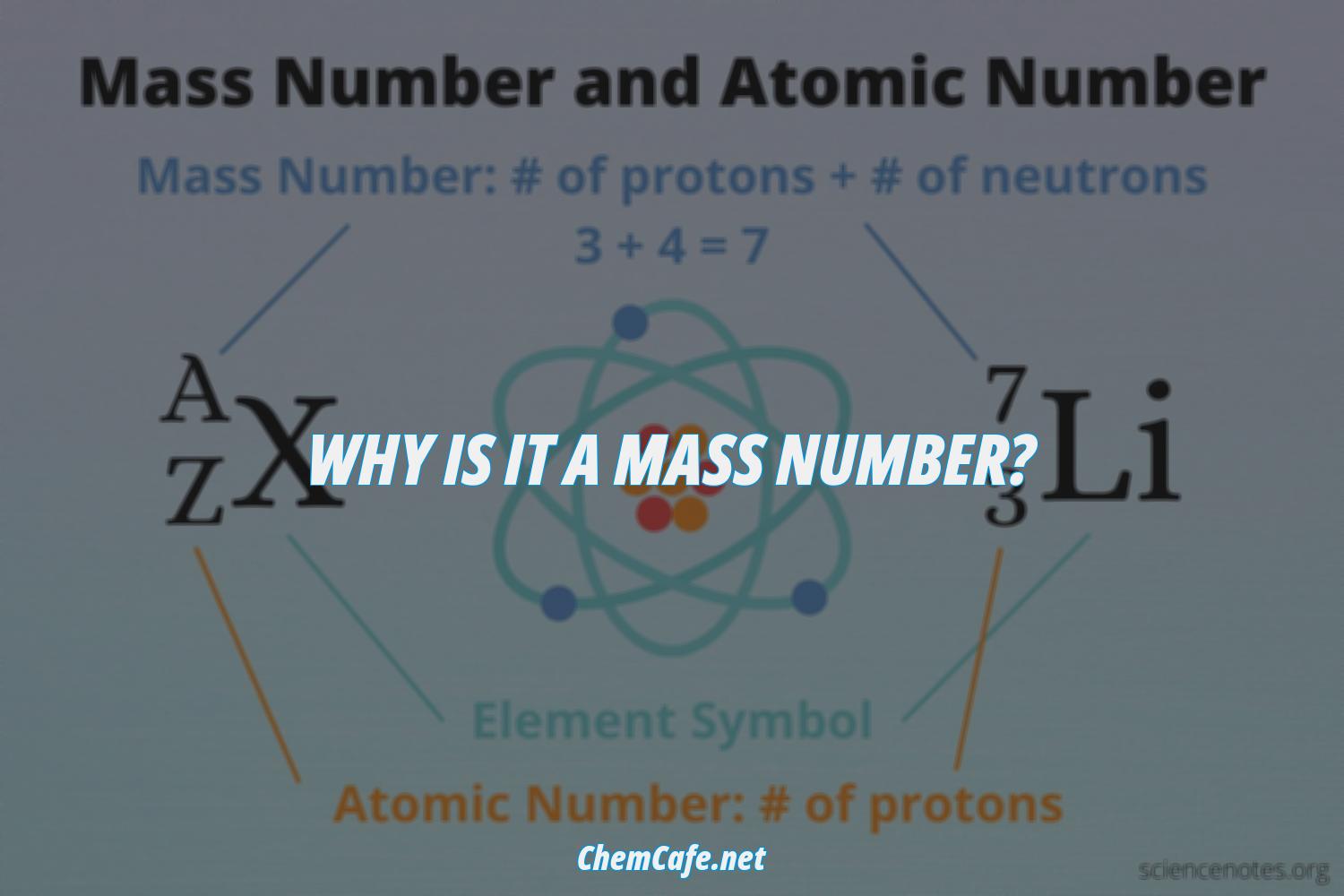
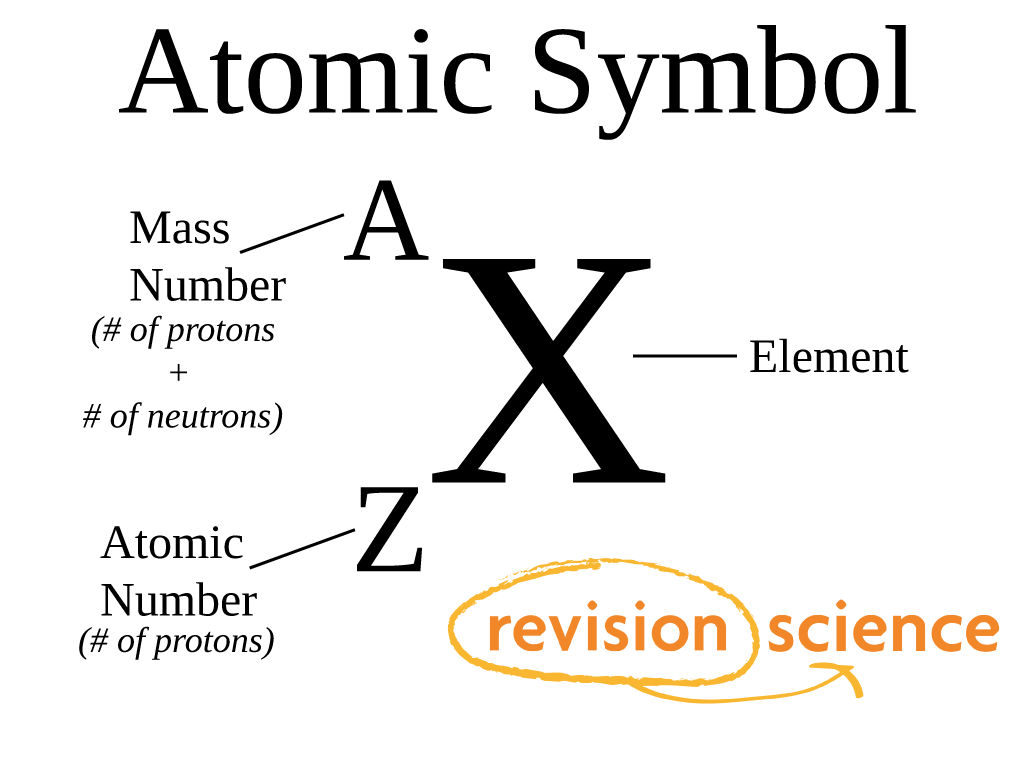
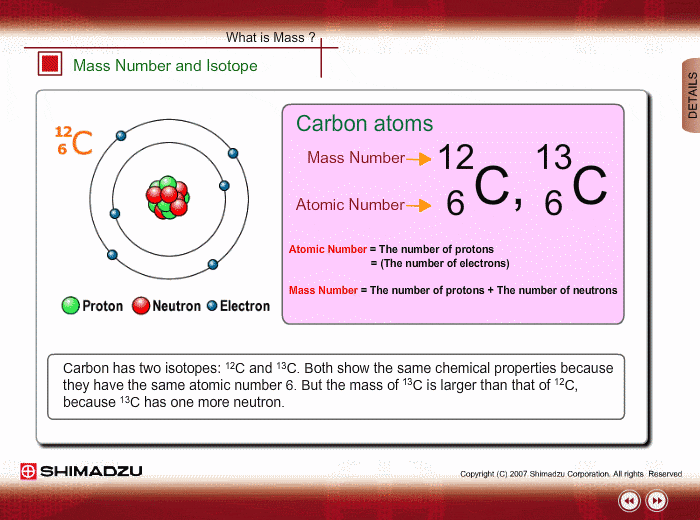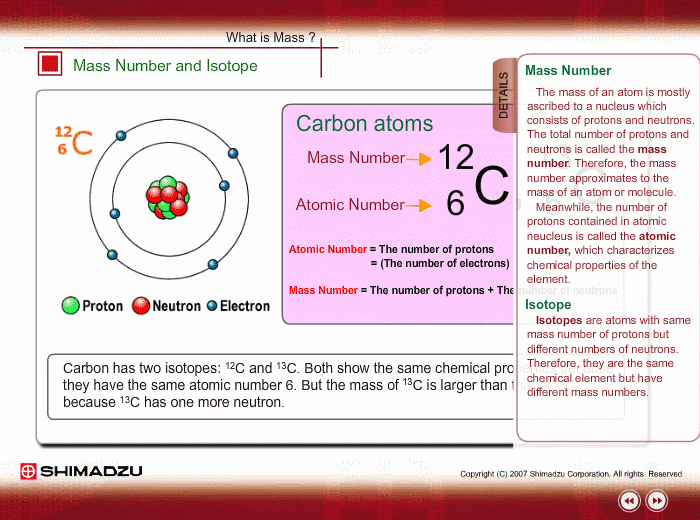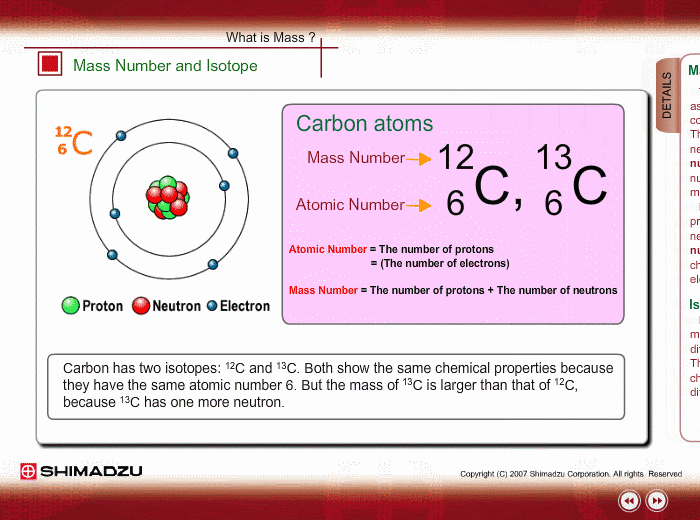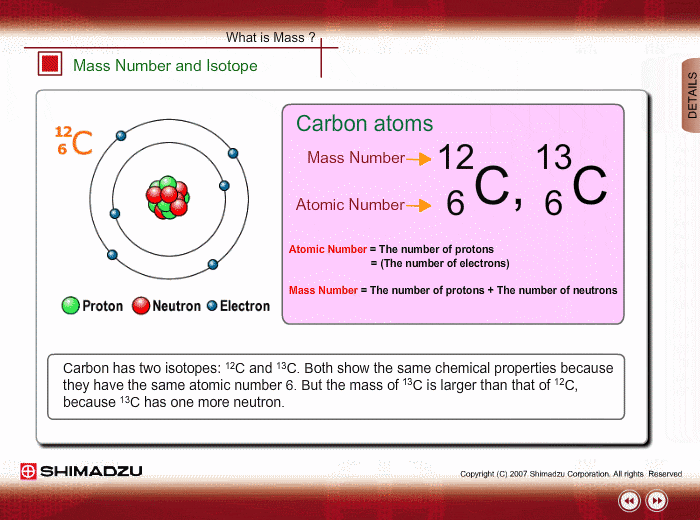
And so for #""^1H#, the mass number is simply 1; for #""^2H#, the mass number is simply 2; for #""^3H#, the mass number is simply 3. The isotopes contain #"0, 1, 2 The mass number of an isotope is the total number of protons and neutrons in an atomic nucleus. If you know that a nucleus has 6 protons and 6 neutrons, then its mass
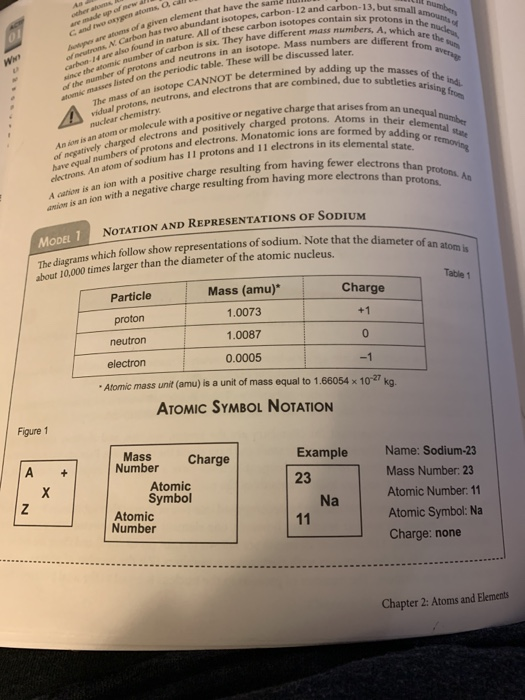
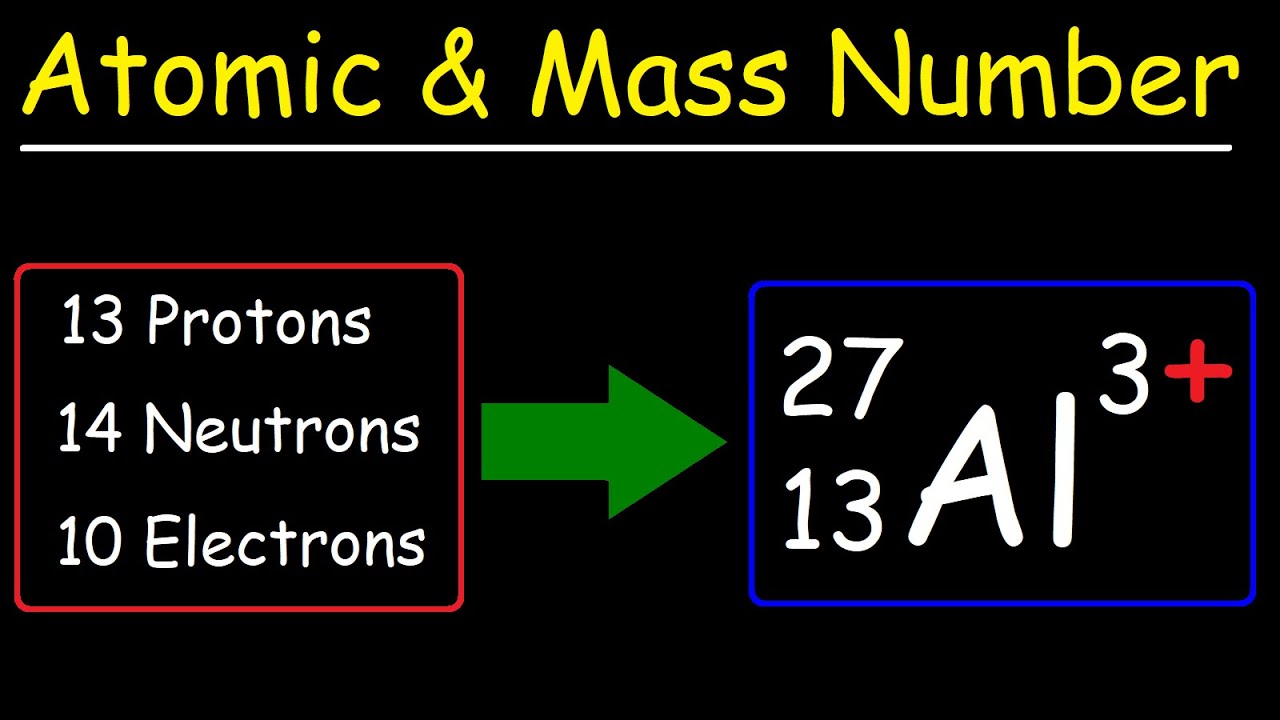
The reason it is called Mass because all the mass of the atom is due to neutrons and protons present in the nucleus of an atom. for example lets take "Carbon".. Mass number is the sum of the protons and neutrons in the atomic nucleus. The mass number ("A") is the sum of the atomic number ("Z"), which is the number of
Related Posts of What Is Mass Number :
And so for #""^1H#, the mass number is simply 1; for #""^2H#, the mass number is
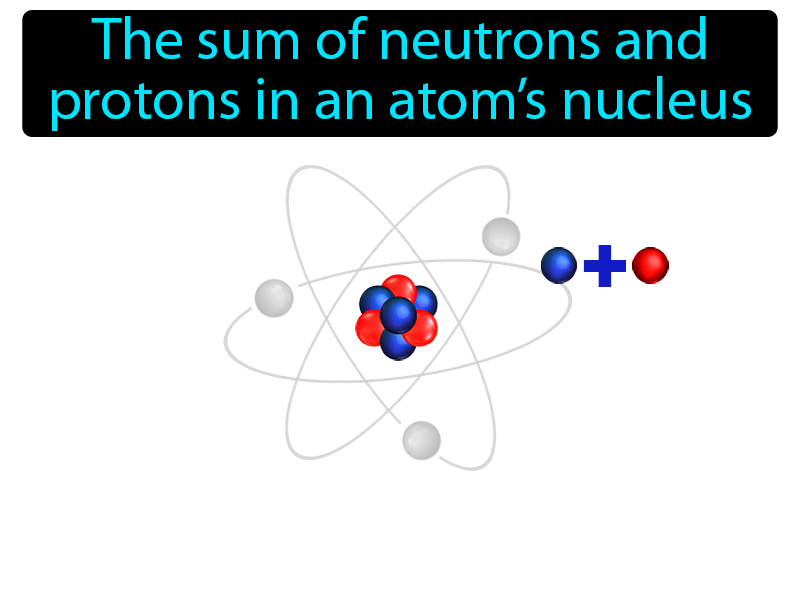
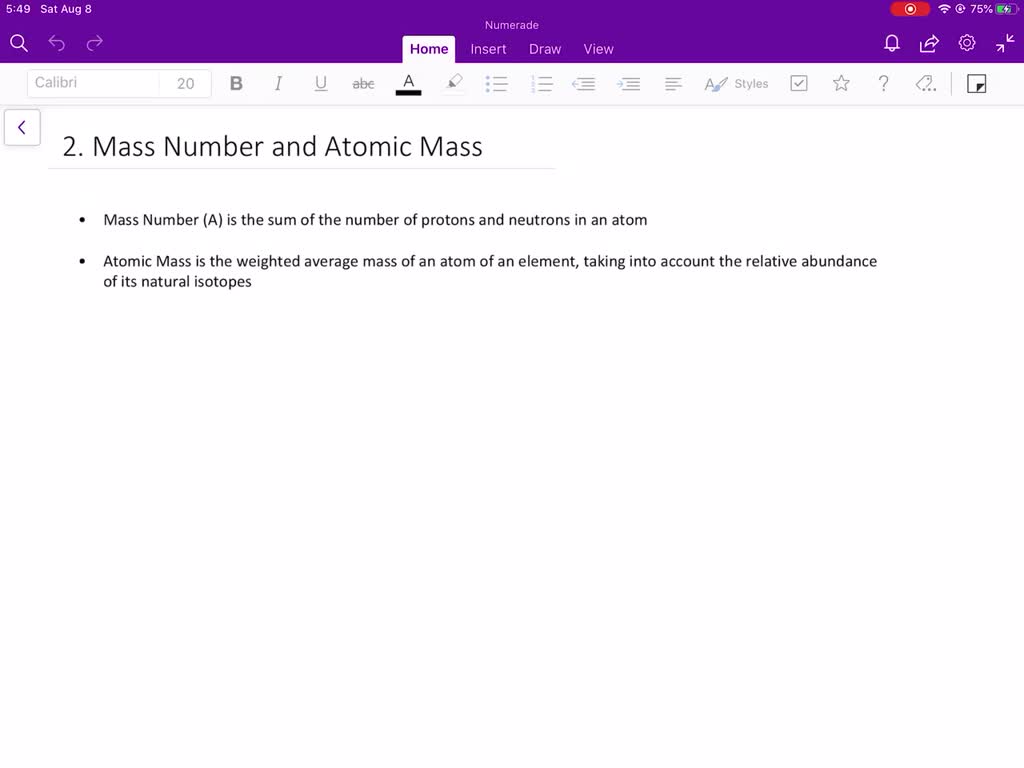
The mass number of an isotope is the total number of protons and neutrons
The reason it is called Mass because all the mass of the atom is due to
Mass number is the sum of the protons and neutrons in the atomic nucleus.
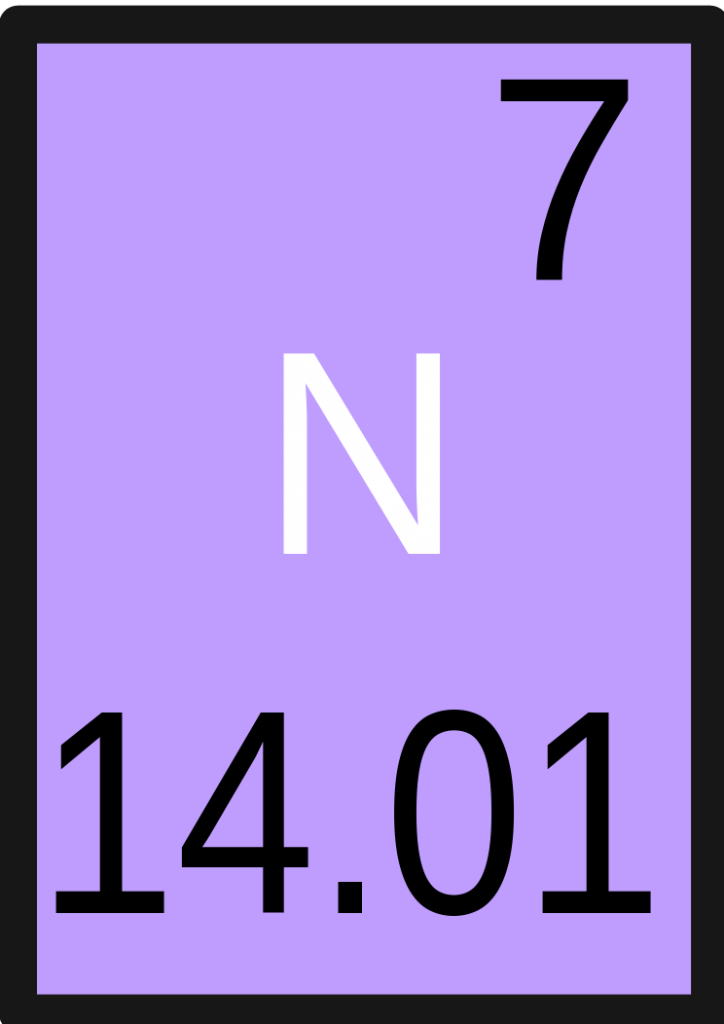
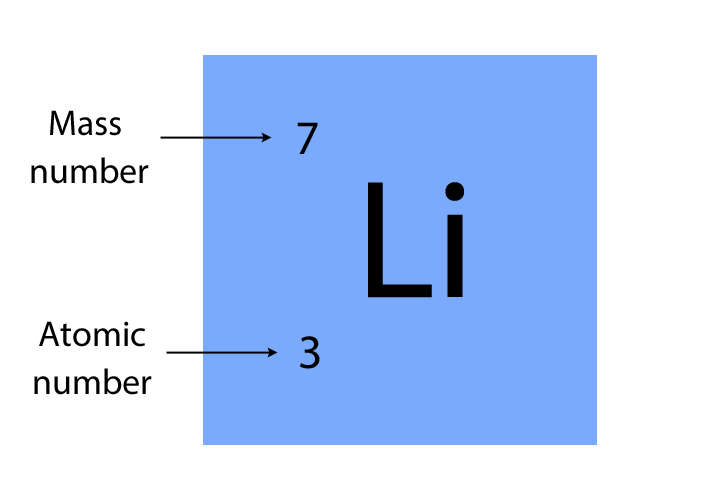
By way of example, the element boron has Z=5, and has an isotopic
Mass Number Example 3 Video Chemistry CK 12 Foundation
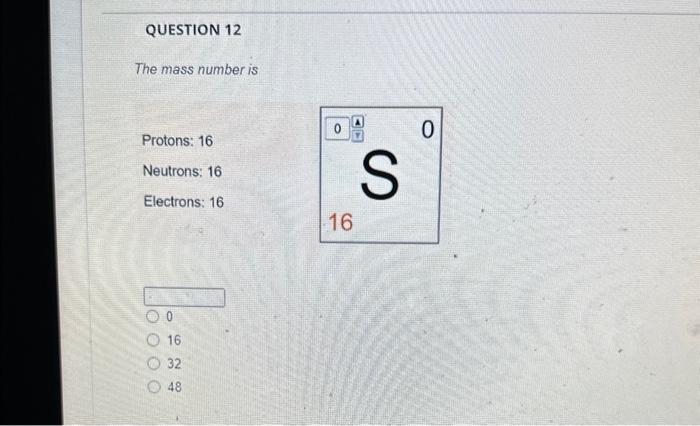
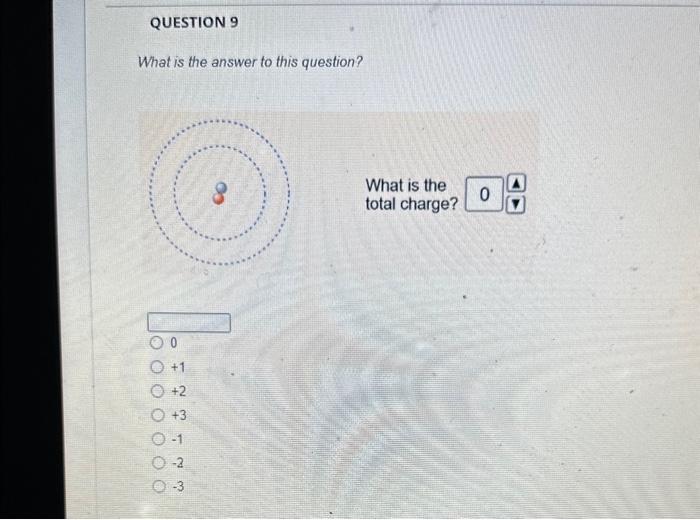
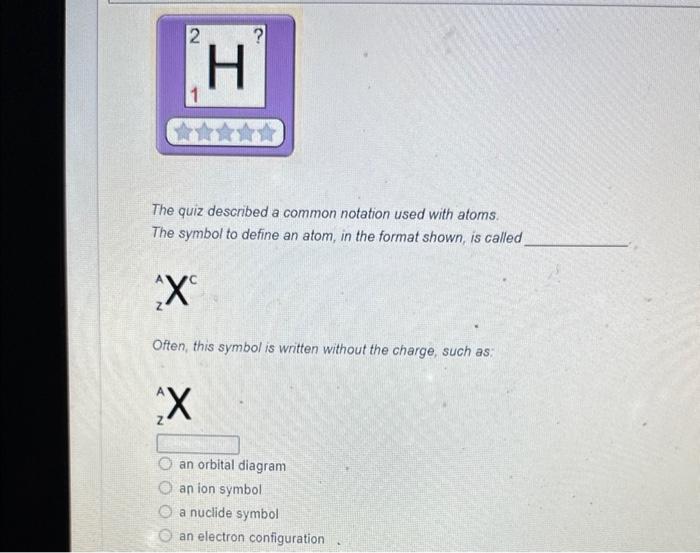
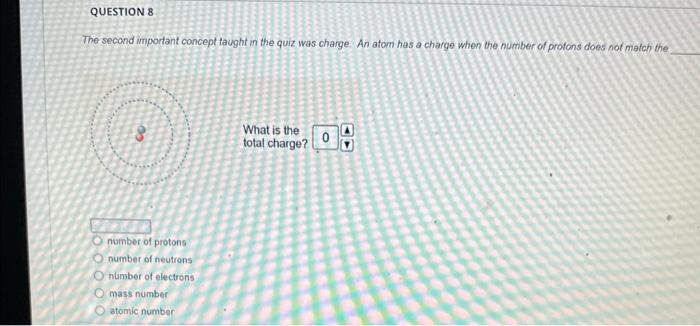
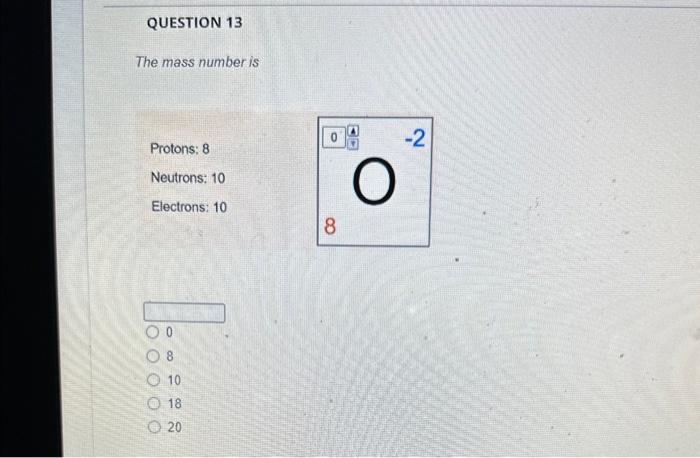
Solved The mass number is What is the mass number The mass Chegg com
Solved The mass number is What is the mass number The mass Chegg com
Solved The mass number is What is the mass number The mass Chegg com
Solved The mass number is What is the mass number The mass Chegg com
Solved The mass number is What is the mass number The mass Chegg com
Solved The mass number is What is the mass number The mass Chegg com
Difference Between Mass Number and Atomic Mass
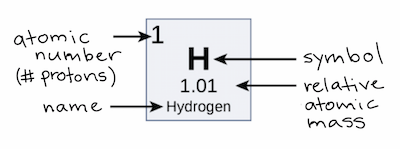
Atomic Number vs Mass Number What s the Difference
Mass Number Example 1 Video Chemistry CK 12 Foundation
Difference Between Mass Number and Atomic Number 88Guru
Mass Number Definition amp Overview Expii
SOLVED Define mass number What is the difference between mass number
Mass Number Versus Atomic Number and Atomic Mass
What Is Atomic Number And Mass Number
Periodic Table Mass Number Meaning Review Home Decor
Mass Number Definition Examples Stages amp Summary
Mass Number Definition Examples Stages amp Summary
Difference Between Atomic Number and Mass Number Definition
Definition of mass number Artofit
Definition of mass number Artofit
Definition Of Mass Number In Science FEDNIT
What is Mass Number amp Defination Examples Popular Gyan
Why is it a mass number ChemCafe science chemistry and physics
Atomic Number and Mass Number A Level Chemistry
How To Calculate The Mass Number
How Is The Mass Number Calculated
Example 2 What is the difference between mass number a whole number On
Solved 7 Given the definition of mass number and the Chegg com
Mass And Atomic Mass Number
Gcse Periodic Table With Mass And Atomic Numbers Cabinets Matttroy
what does the little number mean following an element symbol Wiring Work
Why mass number is represented by A what is mass number Nucleon
mass number definition of mass number applied chemistry 1st
mass number definition of mass number applied chemistry 1st
Mrs Brostrom Integrated Science C ppt download
Chapter 3 Atoms and Elements ppt download
Day 5 Chapter 4 Section 2 and ppt download
Day 5 Chapter 4 Section 2 and ppt download
Unit 2 Matter I hope this is what we were supposed to do please let me
The Structure of Atoms and Ions ppt download
Bellwork or Teacher s Pet Atoms Write 3 facts for the video ppt download
Point System Answering Questions correctly in class 1 pt each answer
STRUCTURE OF AN ATOM BACKGROUND KNOWLEDGE ppt download
Atomic Structure Review Foldable ppt download
Chapter 3 Atomic structure ppt download
Structure of the Nucleus ppt download
Introduction to Atomic Structure ppt download
X Chapter 4 Test Review The Atomic Theory ppt download
Chemistry Basics ppt download
Atomic Structure ppt download
General Vocab General Vocab Atoms Periodic table ppt download
KTQ 4 Orbital Anion Cation Ion Valence Electron Atomic Mass Isotopic
inside the atom lesson 0772 TQA explorer
What Is Mass Number - The pictures related to be able to What Is Mass Number in the following paragraphs, hopefully they will can be useful and will increase your knowledge. Appreciate you for making the effort to be able to visit our website and even read our articles. Cya ~.
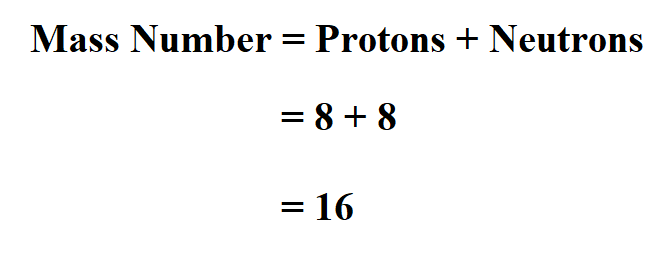







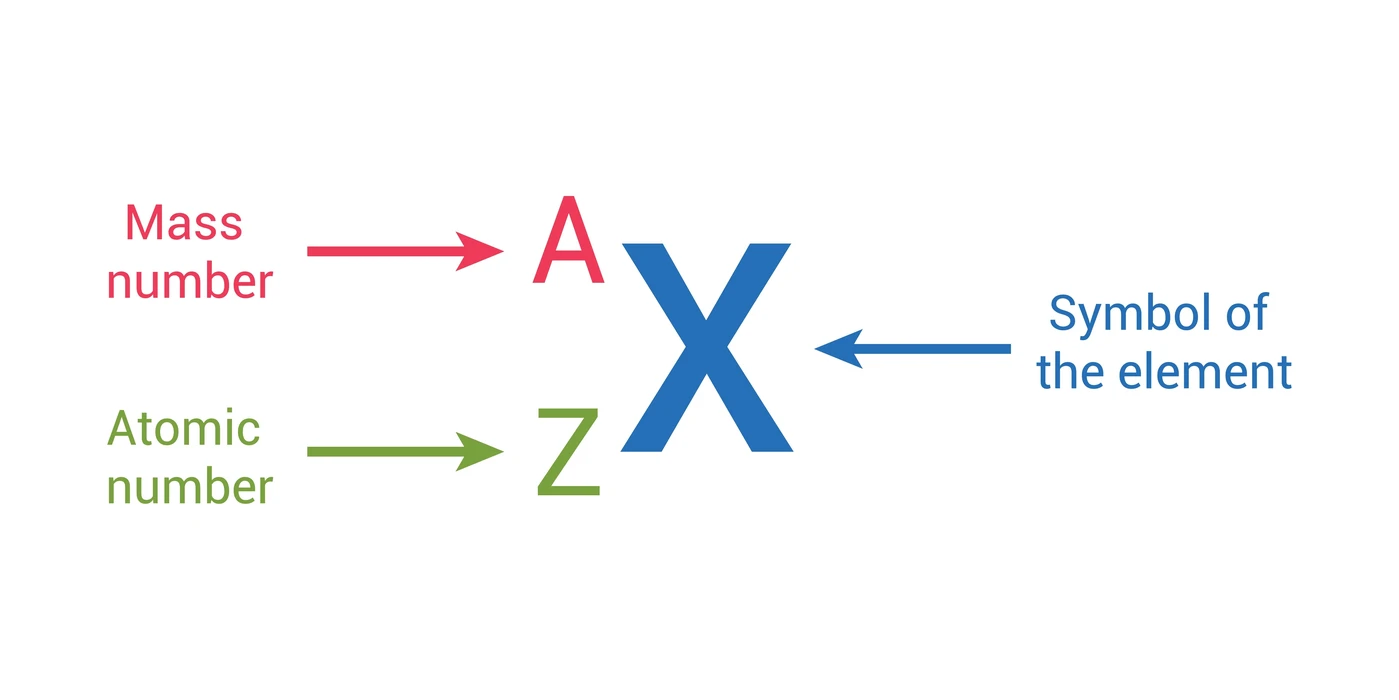


/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
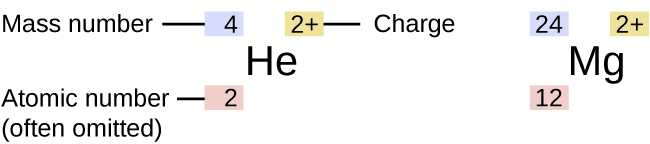





.PNG)




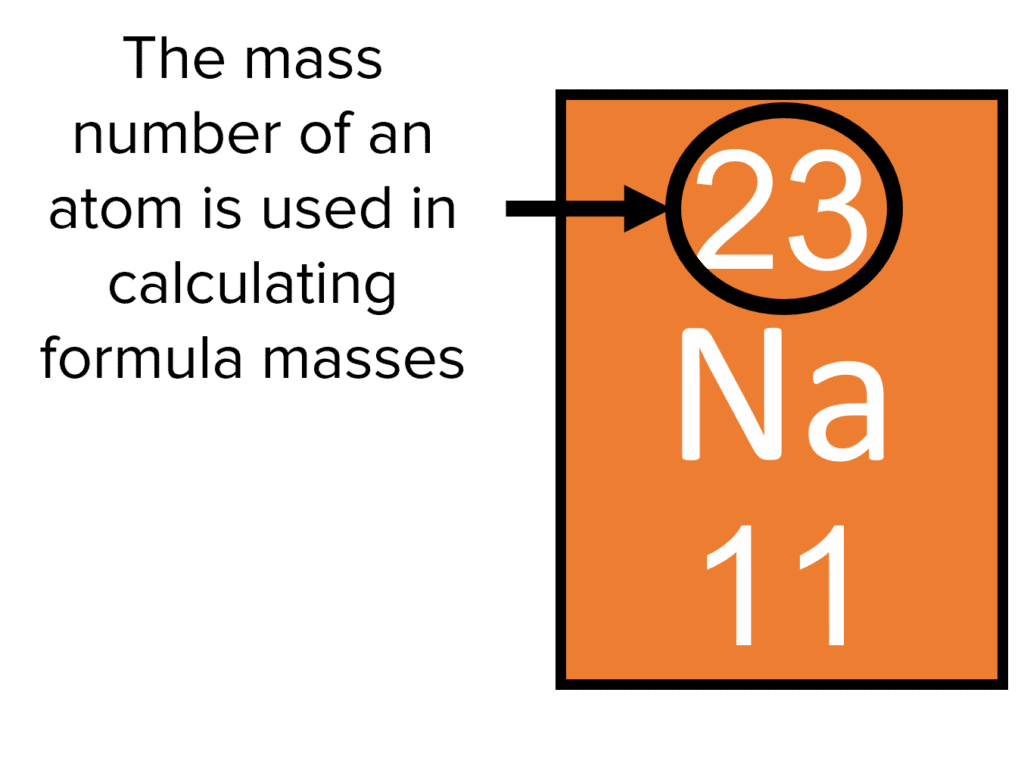
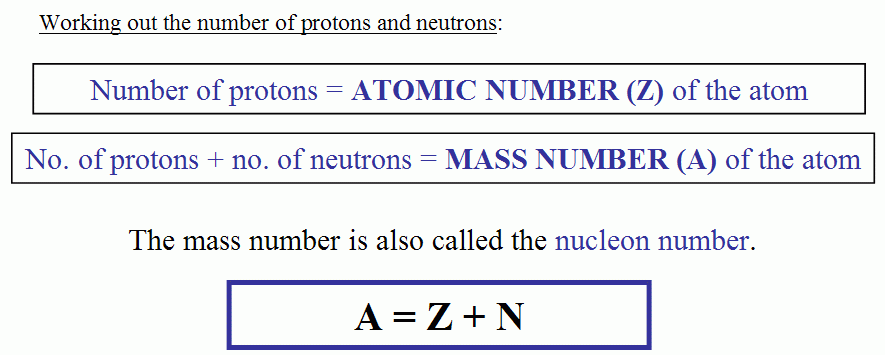








.PNG)

.PNG)
.PNG)
.PNG)





















